|
|
|
Dear
Jacqueline,
Beginning the
New Year with resolutions, we at IRI are planning for our next level
of public service so that we can devote full time to this vitally
important cause of clean energy. IRI is hereby issuing a Call
for Papers for
our next joint Conference on
Future Energy (COFE),
along with several other forums under the auspices of the Natural
Philosophy Alliance, to be held July 11-13, 2013 at the
University of Maryland. As many of you know, we are the only
organization in the US that since 1999 has successfully diagnosed,
educated the public and advocated future
energy innovations
that can truly be called "breakthroughs." IRI wants to
continue this critical service at a more expanded scale of operation
in 2013 so any suggestions, endowments or referrals will be
gratefully accepted. By comparison, I attended "Energy
Innovation 2013" sponsored by the "Breakthrough
Institute" (www.itif.org)
in downtown DC with several panels of speakers, none of whom
represented real innovation nor any breakthroughs, except Kevin
Bullis, the Senior Editor for Energy of MIT's Technology
Review (see
his articles below). I met with him afterwards and gave him a review
copy of my book, Zero Point
Energy: The Fuel of the Future, since as I pointed out,
this emerging energy technology has not appeared in his magazine at
all but it has been featured at many conferences. At Energy
Innovation 2013, there was talk of "zero carbon" emissions
but no one had any idea of how to achieve such a lofty goal but we do
with the IRI white
paper on a national energy plan! Another example of a
political energy overture is the upcoming "Symposium on
Energy in the 21st Century" (April 12, 2013) with
the theme "A Future Using Net Zero Energy" that
seems confusing unless we go back to a Stone Age. Acknowledgement is
given for a technological hopeful from NREL who will be a symposium
speaker, Senior
Engineer Jerry Davis.
Our first FE
eNews story of 2013 is an example of a real breakthrough from Science
magazine with
lots of Supplementary
material online. Using a heretofore unknown principle of a
negative "Gibbs free-energy change of absorbed water"
during an expansion and contraction cycle, a polymer married to a
piezoelectric strip generates up to 1 volt and a fraction of a
nanowatt by simply absorbing water thus converting chemical potential
energy in water gradients to mechanical work!
The second
story is an exciting demonstration
from Finland of
a new light-trapping surface for photovoltaic (PV) surfaces losing
only 8% to transmission instead of the usual 46% from anti-reflective
coatings thus significantly improving efficiency. The third story
from Nature follows
this theme of innovations in solar panels with a sticky panel for any
surface that is lightweight and flexible.
A fourth
article summarizes the results of the US DRIVE (Driving Research and Innovation
for Vehicle Efficiency) government
program of the DOE. The National Academy of Sciences is
critical of replacing petroleum but interestingly, Amory Lovins from www.rmi.org has
the final say with his usually succinct assessment that I was
privileged to receive as a member of a global energy news group which
is optimistic "with RMI's help".
Our last story
is an inspiration for electric car owners since the future seems to
be with lithium-sulfide batteries that are projected by Lawrence
Berkley labs to achieve double the storage capacity of current
lithium-ion batteries which has interested the
ARPA-E program as
wel
Sincerely,
Thomas
Valone, PhD, PE.
Editor
|
|
|
QUICK LINKS
|
|
LIKE US ON FACEBOOK AND GET
10% OFF ANY ITEM IN OUR CATALOG!
 
|
|
|
|
1) Free Energy: Polymer Generator Driven by
Water
|
9:00 10 January 2013 by Jacob Aron- New Scientist
http://www.newscientist.com/article/dn23066-muscle-mimic-pulls-electricity-from-wet-surface.html
Electricity
has been squeezed from a damp surface for the first time, thanks to a
polymer film that curls up and moves - a bit like an artificial
muscle - when exposed to moisture. The film could be used to run small,
wearable devices on nothing but sweat, or in remote locations where
conventional electricity sources aren't available.
 
See Video Click here
When a dry
polymer absorbs water, its molecular structure changes. This can, in
principle, be converted into larger-scale movement, and in turn
electricity. But previous attempts at creating a material powered by
a moisture gradient - the difference in chemical potential energy
between a wet region and a dry region - failed to produce a useful
level of force.
These
unsuccessful tries used a polymer called polypyrrole. Now Robert Langer and colleagues at
the Massachusetts Institute of Technology have turned to the material
again, embedding chains of it within another material, polyol-borate.
This more complex arrangement mimics structures found in muscles as
well as in plant tissues that bend in response to changes in
humidity.
Flipping film
The result
looks like an ordinary piece of thin black plastic, but when placed
on a wet surface, something extraordinary happens. As the material
absorbs water, its end curls away from the surface and the film
becomes unstable, so it flips over. The ends have now dried out, so
they are ready to absorb more water, and the whole process repeats
itself. This continuous flipping motion lets the film travel across a
suitably moist surface unaided.
Langer
found that a 0.03-millimetre-thick strip, weighing roughly 25
milligrams, could curl up and lift a load 380 times its mass to a
height of 2 millimetres. It was also able to move sideways when
carrying a load about 10 times its mass.
To extract
energy from this effect, Langer's team added a layer of piezoelectric
material - one which produces electricity when squeezed. When
this enhanced film, weighing about 100 milligrams, flipped over, it
generated an output of 5.6 nanowatts - enough to power a microchip in
sleep mode.
Electricity from sweat
Though the
output is small, it is proof that electricity can be extracted from a
water gradient. "To the extent of our knowledge, we are the
first to utilise a water gradient, without a pressure gradient, to
generate electricity," says Langer.
Large-scale
energy harvesting is unlikely as the size of the device needed would
be impractical, but it could be used to power small devices such as
environmental monitoring systems in remote locations. "It will
be interesting for applications where the amount of energy needed may
be low but where access to energy may be difficult," says Peter Fratzl at the Max-Planck Institute
of Colloids and Interfaces in Potsdam, Germany, who was not involved
in the work.
Another
application, Langer suggests, would be to place the film inside the
clothing of joggers or athletes. The evaporation of sweat could
generate enough electricity to power sensors monitoring blood
pressure and heart rate.
Journal
reference: Science, DOI
10.1126/science.1230262
back to table of
contents
|
2) How Light-Tapping Surfaces Will Boost Solar
Cells Efficiency
|
|
MIT Technology
Review, January 2013
http://www.technologyreview.com/view/510016/how-light-trapping-surfaces-will-boost-solar-cell-efficiency/?utm_campaign=newsletters&utm_source=newsletter-weekly-energy&utm_medium=email&utm_content=20130121
Improving the efficiency of photovoltaic cells is
one of the great challenges for renewable energy science. In
the lab, the best cells can convert almost half the sunlight hitting
them into electricity (44 per cent) although for the figure
commercial cells is less than half that.
One way to improve matters is to minimise the
amount of light reflected from the cell or transmitted through it,
since this energy is clearly lost. The conventional approach is
to use an anti-reflection coating which can be optimised to minimise
reflections particularly at the cell's optimal frequencies.
But there's a problem. While these coatings are
good at preventing reflections, they cannot stop light being
transmitted. And for the next generation of thin film solar
cells, this is a particular problem. In some cases, almost half the
light passes straight through.
So the most recent research is focused on a
different approach-capturing incoming light and trapping it against
the surface. This prevents both reflection and transmission and
so has the potential to significantly increase the efficiency of thin
film solar cells. The question, of course, is how best to do this in
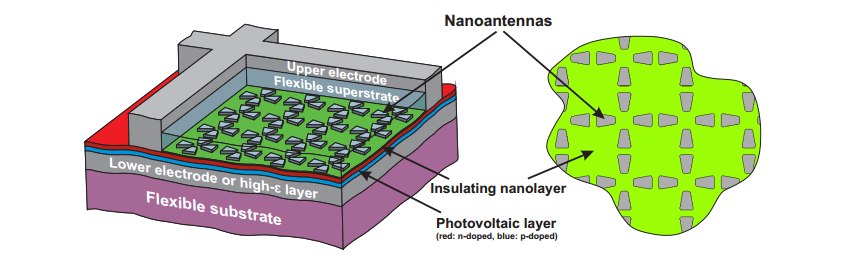
a way that is commercially viable. Today, Constantin Simovski
at Aalto University in Finland and a few pals reveal their design for
a new light-trapping structure. Their idea is to cover a cell with a
regular array of silver nanoantennas that convert ordinary incoming
waves into more exotic ones that propagate through the photovoltaic
slab itself.
The
work is a theoretical study and simulation of how good these
nanoantennas can be and the conclusions are promising. "We
demonstrate that [the nanoantenna array can] increase significantly
the overall spectral efficiency of solar cells with a very small
thickness." they say.
The
simulations produce some interesting numbers. Simovski and co
calculate that an ordinary anti-reflection coating about 7 per cent
of the light is lost due to reflection while 46 per cent is lost to
transmission.
By contrast, their light-trapping
surface loses 20 per cent to reflection but only 8 per cent to
transmission. The extra surface itself absorbs a further 6 per cent.
That's significantly better but there's also the important question
of fabrication costs.
Simovski and co say that new
fabrication techniques for printing a nanoantenna array on a thin
film mean it could be done at low cost. Whether this can be done on
the required scale at a price that is cost-effective, remains to be
seen.
Nevertheless, light trapping
surfaces look a promising way to increase the efficiency of thin film
solar cells-provided somebody can work out how to make them cheaply
enough.
Ref: arxiv.org/abs/1301.3290:
Enhanced Efficiency of Light-Trapping Nanoantenna Arrays for
Thin Film Solar Cells
back
to table of contents
|
3) Flexible
Solar Cells that Stick to Any Surface
|
Kevin Bullis, Technology
Review, January 2013
http://www.technologyreview.com/news/508946/flexible-solar-cells-can-stick-to-just-about-any-surface/?utm_campaign=newsletters&utm_source=newsletter-weekly-energy&utm_medium=email&utm_content=20121224
Solar panels are
typically heavy, which makes them expensive to install, and rigid,
which limits where they can be used. In the current issue of Nature Scientific Reports, researchers
describe a novel, potentially cheap way to make solar cells that are
both lightweight and flexible.
|

|
|
Solar Cell affixed to a Business Card
|
The technique is meant to work with thin-film solar
cells. The active part of thin-film cells-the part that gathers
sunlight and generates electricity-is thin enough to be flexible, but
the cells usually have to be manufactured on rigid materials such as
glass to achieve the highest quality.
Researchers led by Xiaolin Zheng,
a professor of mechanical engineering at Stanford University,
demonstrated a way to transfer the active materials of the solar cell
from a rigid substrate onto another surface, such as a sheet of paper
or plastic, the roof of a car, or the back of a smartphone. As with
other solar cells, wires would then be connected to deliver power,
but flexible solar cells could be used on curved surfaces, and,
because they're lightweight, they would be easier to install than
conventional panels.
Although Zheng has
demonstrated that the process can transfer solar cells even to cheap
surfaces such as paper, in practical applications, the materials used
would be limited by the need to protect the cells from the elements.
These aren't the first flexible solar panels. Several
companies already manufacture them (see "A Solar Startup that
Isn't Afraid of Solyndra's Ghost" and "Solar Shingles See
the Light of Day"). But Zheng says prior approaches
to making flexible solar cells have drawbacks. Manufacturers often
modify processing steps to accommodate flexible substrate materials
that can't tolerate high temperatures or certain chemicals, but this
can reduce the performance of the resulting solar cell. And
manufacturers have typically used costly flexible substrate
materials, such as foils with extremely uniform surfaces, in order to
produce high-quality thin films.
The trick to peeling
thin-film silicon away from a solid substrate of silicon dioxide
involves depositing a layer of nickel on top of an underlying wafer.
After the cell is finished, it's immersed in room-temperature water.
The water interacts with the nickel and silicon dioxide, causing the
solar cell to come loose. It can then be peeled away and deposited
onto another material. The researchers demonstrated that the
efficiency of the solar cell wasn't affected by the transfer process.
The current paper shows that
the process works for dislodging a solar cell from a silicon and silicon
dioxide wafer. Zheng says the group has also demonstrated the process
with solar cells made on a glass surface, but this work has not yet
been published. This could make it possible to use the technique with
copper indium gallium selenide solar cells, which are nearly twice as
efficient as amorphous ones.
|
4) Review of
US DRIVE Partnership
|
|
Lorin Hancock,
Jan. 23, 2013, National Academy of Sciences
(Ed. Note:
scroll down to Amory Lovins' solution to a complex political problem
cited in this press release! - TV)
FOR IMMEDIATE
RELEASE 
WASHINGTON -- A new report from
the National Research Council calls the operation and management of
the technical teams of U.S. DRIVE generally "exemplary,"
but finds that its Executive Steering Group has not provided adequate
guidance for fitting the technical teams' work into an overall plan
for the partnership's goals
of reduced petroleum use. The public-private partnership
has made steady progress in creating viable alternatives to
gas-powered vehicles, but formidable
technical barriers have
prevented the emergence of a stand-out contender to replace petroleum.
U.S. DRIVE is a
government-industry partnership conducting precompetitive research
and development to help accelerate the emergence of advanced
technologies for clean and efficient light-duty vehicles that could
eventually compete commercially with petroleum vehicles. The
partnership participants include four automotive companies, five
energy companies, two electric power companies, and the Electric
Power Research Institute, with the U.S. Department of Energy
providing federal leadership. The partnership has determined
three potential primary pathways
to reaching significantly reduced petroleum consumption: improved
internal combustion engine vehicles coupled with greater use of
biofuels and natural gas in conventional or hybrid vehicles; expanded
use of plug-in hybrid electric vehicles and battery electric vehicles;
and the possible transition to hydrogen as a transportation
fuel. Nine technical teams, including those on hydrogen
storage, grid interaction, and combustion and emissions control,
focus on specific research needed to make any or all of the pathways a
commercial possibility.
As in previous
Research Council reviews of the FreedomCAR and Fuel Partnership --
predecessor of U.S. DRIVE -- the report finds the operation and
management of the technical teams and the integration of the systems
analysis functions within those teams to be exemplary for the most
part, and provides recommendations in specific technical areas.
However, it is not apparent that critical issues being investigated
by the technical teams are guided and prioritized by an overall understanding
of how these technical improvements affect larger program
goals. Without overarching guidance, there is a potential for
conflict among the respective goals of the various technical
teams. It is imperative that the partnership's Executive
Steering Group, Joint Operations Group, or other program
decision-making groups continually broaden their understanding of
these implications and adapt research plans to provide effective
portfolio management.
The Executive
Steering Group should set targets for the technical teams that are
consistent with the objectives of reduced
petroleum consumption and greenhouse gas emissions,
and U.S. DRIVE should conduct a comprehensive review of the
partnership's portfolio, the report says. Focusing on the
mission of supporting longer-term, higher-risk precompetitive
activities in all three potential primary pathways, the review will
ensure that research and development efforts are adequate and
appropriate to achieve the targets. The report also recommends
adopting a portfolio-based research and development strategy to
balance the investment among alternative pathways with more
traditional reviews of the individual pathways' progress.
The
study was sponsored by the U.S. Department of Energy. The
National Academy of Sciences, National Academy of Engineering,
Institute of Medicine, and National Research Council make up the
National Academies. They are private, independent nonprofit
institutions that provide science, technology, and health policy
advice under a congressional charter. The Research Council is
the principal operating agency of the National Academy of Sciences
and the National Academy of Engineering.
RELATED
COMMENTARY AND SOLUTION
From: On
Behalf Of Amory
B. Lovins
Sent: Wednesday,
January 23, 2013 2:29 PM
To: Global
Energy Network
Subject: Re:
[global-energy] FW: New Report: Review of U.S. DRIVE Partnership
The problems referred
to have a common cause and a common solution. They can be resolved by
a different vehicle-design strategy starting with platform
"fitness" - taking the obesity (unnecessary weight and
drag) out of the automobile. This not only saves about two-thirds of
the tractive load (the energy required to move the auto), at
approximately zero net marginal manufacturing cost, but also makes
affordable the advanced powertrains, specifically electrification,
that can displace the rest of the fuel. This strategy is described in
Chapter Two of Reinventing
Fire (www.reinventingfire.com,
2011). It confers strong competitive advantage on early adopters; is
already being adopted by some automakers, often with RMI's help, and
is in discussion with others; and has been encouragingly received by
DOE leadership.
Amory B. Lovins
Chairman and Chief Scientist
Rocky Mountain Institute
1739 Snowmass Creek Road
Snowmass CO 81654, USA
ablovins@rmi.org
back to table of contents
|
5) Lithium-sulfide
Batteries could store far more Energy than Lithium-ion
|
Kevin Bullis,
Technology Review, January 2013
http://www.technologyreview.com/news/508466/two-advances-point-toward-a-cheaper-electric-car-battery/?utm_campaign=newsletters&utm_source=newsletter-weekly-energy&utm_medium=email&utm_content=20121224
For the
roads to start filling up with electric cars, batteries will need to
get much cheaper-as much as 80 percent cheaper by some estimates (see
"How Improved
Batteries Will Make Electric Vehicles Competitive").
Two recent advances that make an experimental type of battery much
more practical could lead to such cost savings.
Researchers
have for years been working on a type of battery that uses lithium
metal in one electrode and sulfur in the other. In theory, this kind
of battery could store three to five times as much energy as a
conventional lithium-ion battery (see "Revisiting
Lithium-Sulfur Batteries"). But lithium metal is
highly reactive when exposed to water and can form root-like
structures inside batteries over time; these structures can join
positive and negative electrodes, causing short circuits and even
fires. So many researchers have begun turning their attention to a
similar battery that doesn't require lithium metal.
In the new type of battery, the sulfur
electrode is replaced with a lithium-sulfide material-a compound that
contains both lithium and sulfur. This becomes the source of the
lithium, so the lithium metal is no longer required and can be
replaced with graphite-a material used in lithium-ion batteries
today-or with a material such as silicon.
The trouble is, lithium sulfide is electrically
insulating, which slows down charging and reduces the amount of
energy the battery can deliver. But two recent papers, one from
Stanford and the other from Lawrence Berkeley National Laboratory,
offer ways to make lithium-sulfide batteries more practical.
These
research papers demonstrate low-cost methods for making
lithium-sulfide batteries with high-energy storage capacities. The
work could lead to commercial batteries that store more than three
times as much energy as the lithium-ion batteries currently used in
electric vehicles, says Yuegang Zhang,
a staff scientist at Lawrence Berkeley National Laboratory.
Earlier this year, Yi Cui, a materials science
professor at Stanford, showed a way to overcome the inherent
limitations of lithium-sulfide batteries by charging the battery at a
higher voltage than usual for its first charge. This changes the
chemistry of the electrode, getting around the conductivity problem.
Even then, the lithium sulfide had to be mixed
with carbon to improve its conductivity, and the carbon decreases the
amount of energy the electrode can store: in experiments, it was
enough to reduce the battery's capacities to levels close to
conventional lithium-ion batteries.
Zhang demonstrated a new way to mix the carbon
with the lithium sulfide that greatly reduces the amount of carbon needed
in the cathode. The percent of the electrode that's made up of
lithium sulfide increases from less than 50 percent to 67.5 percent.
This improvement, in part because it's amplified by improvements it
allows in other parts of the battery, could nearly double the overall
battery storage capacity, from 350 to 610 watt-hours per kilogram,
Zhang estimates. (Lithium-ion batteries in electric vehicles now
typically store less than 200 watt-hours per kilogram.)
Obstacles remain to commercializing the technology,
including the need to improve the number of times the batteries can
be recharged and the speed with which they can be charged. The energy
storage numbers should also be taken with a grain of salt-they're
estimates derived from lab-scale experiments, not measurements of
large, commercial-scale batteries.
And
lithium-sulfur batteries that use lithium metal may yet prove to be
the technology of choice. Researchers and companies such as Sion
Power and Polyplus are making progress on improving the number of
times they can be recharged, and are using ceramics or other
materials to address safety issues (see "Beyond Lithium Ion:
ARPA-E Places Bets on Novel Energy Storage").
|
About Integrity Research Institute
Future Energy eNews is
provided as a public service from Integrity Research Institute,
a Non-Profit dedicated to educating the public on eco-friendly
emerging energy technologies.
FREE copy
of the 30 minute DVD "Progress in Future Energy" is
available by sending an email with
"Free DVD" in subject and mailing address in
body.
Your generous support is
welcome by making a tax deductible donation on
our secure website
|
|
|
|
- Scott Kelsey, Missouri State, explaining Rejuvamatrix, Pulsed EMF
therapy to increase the length of DNA telomeres, which directly
affect our lifespan.
- Max Formitchev-Zamilov, Penn State, discussing Cavitation Induced
Fusion, that will soon provide power generation and heat
production.
- Christopher Provaditis, from Greece, explaining Inertial
Propulsion and who teamed up recently with Boeing for their space
satellites.
- PJ Piper of QM Power, discussing the motor invented by
Charles Flynn, with a revolutionary parallel path that gives
double and triple efficiency.
- Dr Thorsten Ludwig from Germany (GASE) discussing
the mysterious Hans Coler motor that WWII British Intelligence
researched.
|
|
|
|
|
|